Heat and mass are transferred in practically every process and event around us. Whether it is boiling water for an afternoon cuppa, melting a piece of ice you have in your drink, or microwaving your late dinner.
Heat and mass transfer are also often inseparable. Think about it, what is being transferred when you:

Image: Apple pie
- take out a hot apple pie from an oven? Hint: can you feel that air around it becomes warm? Can you smell it?

Image: Tea and cream
- put cream to your tea (or coffee, if you anywhere else than the UK)? Hint: you probably store the cream in the freezer, so it is safe to assume it was cold while the beverage was hot. Also, can you notice any changes in the colour?

Image: Hot shower
- take a hot shower? Hint: you probably thought about flowing water, but how about all that generated steam?
Heat and mass transfer are important in everyday life but are also at the core of all manufacturing processes. For example, all chemical reactions require reactants to be delivered to the place of reaction, and products to be taken away – clearly, some mass transfer needs to happen, or we will see no reaction or reaction that stops very quickly. Heat transfer is also important – reactions may need some energy input to happen or may release
energy. If heat is not being efficiently transferred, again, reactions will stop or, in contrast, release so much energy that the situation can lead to a thermal explosion!
Challenge! Can you think about 5 specific processes either in industry or in everyday life where we want to control heat and mass transfer?
Some examples for you, but you can include many more: making ice cream, producing polymers, for example, nylon, running nuclear reactors (did you know that submarines have small nuclear engines?), pasteurising food to kill germs, extracting heat from your laptop, preventing the creation of pollutants.
So how do we control heat and mass transfer? Firstly, we need to look into fundamental principles to understand all the possible mechanisms. You can find more information in the provided resources.
https://www.youtube.com/watch?v=YK7G6l_K6sA
https://www.youtube.com/watch?v=B0CwRyBlt1s
Conduction
A piece of a stainless steel rod is heated on one end until it becomes red. You take it from another end – currently cold. What do you expect will happen as time passes? The end of the rod that you hold will slowly become warmer and warmer, going beyond the point where it will start to burn your hand, and you will need to drop it.
The reason why one end of the rod starts to heat up was given by Isaac Newton in 1701 when he was in Cambridge. Newton said that heat would be transferred from a hot object to its surroundings as long as they have different temperatures. While this statement concerns an object and its surroundings, it as well explains the fundamental reason for heat transfer - and this is the temperature difference. So in our case, even though we have
only one rod, its temperature is different at ends, so heat transfer happens in
the rod itself, through its material – steel.
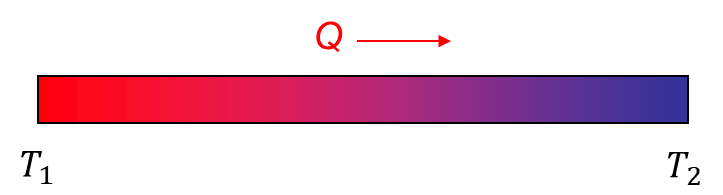
The heat transfer mechanism through a solid material is called conduction and was for the first time mathematically described by Joseph Fourier in 1822. Following Fourier, we can calculate how much heat is being transferred in a unit of time (so per second):
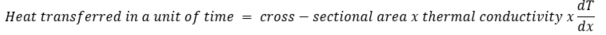
or in a mathematical form:
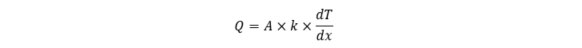
where Q is the rate of heat transfer, A is the area, k is the thermal conductivity, dT denotes the temperature difference (the reason for heat transfer), which we observe at the distance dx.
Here, the thermal conductivity represents the property of the material that allows it to transfer heat. So how do materials do that, and why some transfer heat quickly (think stainless steel rod), while others not (Styrofoam). As it turns out, materials transfer heat through vibrations, movement and collisions of atoms. In metals, the atoms in
the hot region vibrate quickly, affecting neighbours, so passing some energy
further. In gases, molecules are far away from each other, so energy can be
passed only if one molecule collides with another. Hence, stainless steel is a
good heat conductor, while Styrofoam with plenty of air in its porous structure
is a bad heat conductor.
Once we know how quickly heat is transferred through materials, we can start designing some control techniques.
Think about it – what could we do with the rod situation to transfer heat faster or slower?
A simple example.
A study room has one external wall measuring 5 m x 3 m, and is made of 30 cm thick clay brick, for which thermal conductivity is 0.7 W m-1 K-1. Now, we use a hollow brick of the same thickness but thermal conductivity of 0.3 W m-1 K-1.
How does this influence the rate of losing heat from the room at 20º to the outside at 5ºC?
Let’s go through this together.
In the situation with the regular clay brick, we calculate that

In the situation with the hollow brick,
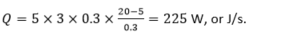
What it means practically is that in the first case, we need to constantly provide 750 W of heat to the room, for example, from radiators, to keep the temperature in the room constant. If we use the hollow brick, we only need to provide 250 W.
Why is silver cutlery not practical?
In the 17th century, the most fashionable cutlery was made of silver. Nowadays, we use much cheaper stainless steel. But the reason for switching to steal was also more practical. The thermal conductivity of silver is 430 W m-1 K-1, and it is one of the largest values found in metals! What it means is that silver conducts heat very quickly, and if you use it for eating piping hot food or stirring hot tea - your fingers will quickly feel the incoming heat! Stainless steel, on the other hand, has a k of 14 W m-1 K-1. In comparison to silver, it will conduct 30 times less heat per second, preventing us from burning our fingers.


