Some examples of nanoscale objects are the following:
• One double strand of DNA has a diameter of around 2.5nm
• Fingernails grow at around 1nm/s
• One virus cell is approximately between 20-400 nm in size.
Many of our core biological entities are on the nanoscale, and so why don’t we start looking at this scale? After all, biology has evolved for billions of years, and our bodies have been fine tuned to be as efficient and capable as possible. Therefore, it is crucial to start looking at individual cells and molecules on the nanoscale compared to the bulk ‘macroscale’, as it gives unique insights and properties which can be used to solve difficult problems.
One of the main reasons that properties are different at the nanoscale is an increased surface area to volume ratio.
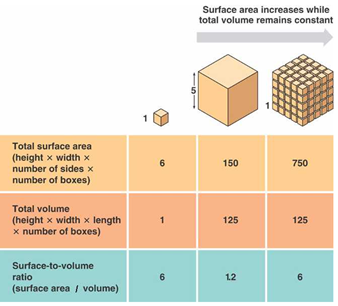
The smaller the cubes, the higher your surface area to volume ratio for the same total volume of material. This means that there is more surface available for reactions to happen on. It is no surprise, therefore, that on the nanoscale, many materials are more reactive. For example, (do not try this at home!), if you throw some very finely ground sugar in the air, and the particles disperse, they become a lot more reactive and if a flame is added, will explode. The larger surface area to volume ratio does not just make things more explosive, but also means things heat up and cool down quicker.


